Clean Room Classifications For Medical Devices
American cleanroom systems modular clean room walls made of frp reinforced plastic and hpl high pressure laminate are often used in pharmaceutical usp 797 compounding rooms and medical device clean rooms. A limited specialty is a specialty contractor classification limited to a field and scope of operations of specialty contracting for which an applicant is qualified other than any of the specialty contractor classifications listed and defined in this article.
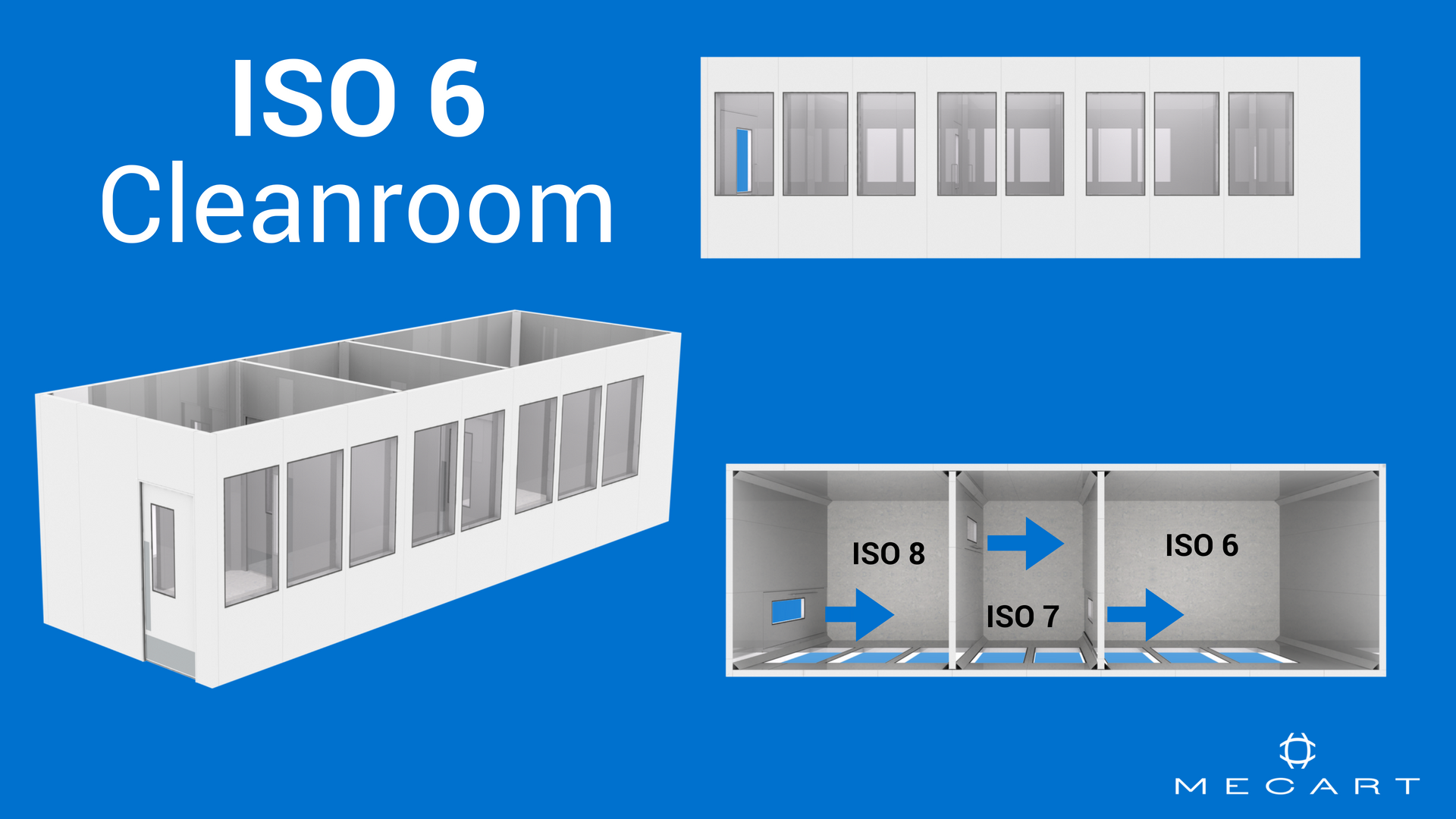
Cleanroom Classifications Iso 8 Iso 7 Iso 6 Iso 5
We are able to supply specialist mopping systems in addition to cleaning tools that have been designed to clean isolators walls ceilings curtains and awkward to reach areas.

Clean room classifications for medical devices. Posted february 2 2018 by bmp medical. Nysed approved 4 contact hour training covers infection prevention infection control practices and procedures barriers ppe safe environment principles and preventing transmission of infectious disease to and from healthcare workers. State of california.
Cleanrooms are classified according to the cleanliness level of the air inside them. Cleanroom disinfection cleaning. Food and drug administration fda.
Cleanroom direct are able to offer a total cleanroom disinfection and cleaning solution. The cleanroom class is the level of cleanliness the room complies with according to the quantity and size of particles per volume of air. If you are into the medical industry or if your work nature is into the commercial medical or industrial industry then you should know about the clean room where every medical industry must work under clean conditions as an extremely small dust particle or off gassing can make the.
Modular cleanrooms iso class 5 clean benches and pass thru cabinets and anti static modular cleanrooms and cleanroom products. The gateway to up to date information on integrated whole building design techniques and technologies. Pumps are not provided for this room.
Clean air products provides clean room solutions for manufacturing medical and pharmaceutical industries including. The goal of whole building design is to create a successful high performance building by applying an integrated design and team approach to the project during the planning and programming phases. New york state mandated infection control course.
Whats the difference between the fda medical device classes. The following guidelines have also been developed to assist departments considering creating additional lactation rooms within their respective buildings. All medical devices sold in the united states are regulated by the us.
So you are looking for iso 14644 for a clean room. Request a quote on cleanroom equipment today.
Clean Room Design And Validation Tsquality Ch
Automotive Cleanrooms Modular Cleanrooms By Total Clean Air

0 Response to "Clean Room Classifications For Medical Devices"
Post a Comment