Clean Room Classification Pdf
Page 1 of 37 environmental monitoring of clean rooms in vaccine manufacturing facilities points to consider for manufacturers of human vaccines. Its the first system of its kind to offer complete isolation between different classes of clean rooms when transferring multiple lines of fluid through a single stainless steel pass through wall portal.
Cleanroom Cabinent Fabrication Stailness Steel Storage Carts And
Clean room.
Clean room classification pdf. Clean room monitoring regulatory standards air classification as per schedule m grade maximum permitted number of particles m3 equal or above. Wmi61 maryland heights mo. A room or area with defined environmental control of particulate and microbial contamination constructed and used in such a way as to reduce the introduction generation.
Glossary 1 xcleanroom. Advantapass is a patented system that permits the aseptic transfer of fluids between the walls of pharmaceutical manufacturing suites. Basic clean room requirements what is a clean room.
By ir liew huey chyan peng apec eng asean eng miem mieaust smiest mashrae. Federal standard 209e for cleanroom an obsolete document. A clean room in my mind are a combination of engineering design fabrication finish and operational controls control strategy that are required to convert a normal room to a clean room.
A cleanroom or clean room is a laboratory facility ordinarily utilized as a part of specialized industrial production or scientific research including the manufacture of pharmaceutical items and microprocessors. Murray street liberty mo 64068 phone.
Certified Clean Room Shoe Covers Clean Room Shoes Usa
Annex 5 Supplementary Guidelines On Good Manufacturing Practices For
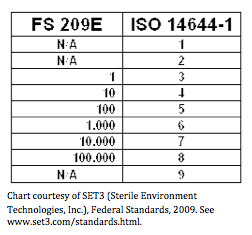
0 Response to "Clean Room Classification Pdf"
Post a Comment